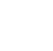10 Analytical Methods
10.1 Introduction
A critical component in establishing soil background, whether it be default or site-specific, is to ensure that the soil samples are analyzed by laboratory methodologies that generate high-quality analytical data that meet the data quality objectives (DQOs) of the soil background study and are comparable to the site data being evaluated. Soil sample concentrations reported by the laboratory can be influenced by the soil sample collection and preservation methods, laboratory sample preparation methods (this includes soil sample preprocessing, digestion, or extraction), and laboratory analytical methods used.
When using data from an existing study to establish soil background, laboratory sample preparation and analytical test methods that were used in the existing study should be evaluated to ensure that they provide substantively equivalent results to the laboratory method used at the investigative site(s) being evaluated. Different jurisdictions may have various definitions of what “substantively equivalent” means. However, it generally means that the two test methods being compared give results for the contaminants being analyzed in certified or standard reference materials that have a small allowable bias between the reported results for the two test methods. The magnitude of the allowable bias can vary by test method and jurisdiction.
Data generated using different laboratory methods may (or may not) be comparable. In cases where there is a need to use data analyzed using different laboratory methods, it is important to evaluate the potential difference between the results generated by the two methods, clearly understand the uncertainties involved, and consider this in risk assessment results and risk management decisions.
When conducting a study to establish soil background, to ensure comparability, it is important that soil samples collected from the area considered background and the area being evaluated are collected and preserved using the same techniques and analyzed using the same sample preparation and analytical test methods (or sample preparation and test methods that provide substantively equivalent results) for each analyte or analyte group. If practical, when conducting a site-specific background evaluation, site and background samples should be analyzed by the same laboratory (and if at all possible, in the same analytical batches) to reduce the potential for test method bias between the site and background datasets.
In some cases, the background dataset is compiled from multiple existing soil background studies. In these cases, the different source datasets should be examined to determine whether they were generated using sample collection, preservation, preparation, and analytical methods that provide substantively equivalent results. Datasets generated from sample collection, preservation, preparation, or analytical test methods (or a combination of any of these factors) that provide substantively different results should not be included in a compiled background dataset. Even if the sample collection, preservation, preparation, or analytical test methods used for different datasets provide substantively similar results, a compiled background dataset from multiple sources should not be created until such a grouping is demonstrated to be technically acceptable using statistical methods discussed in Section 11 and geochemical evaluation methods discussed in Section 5.
10.2 Obtaining Reliable Analytical Data
10.2.1 Data quality
Choosing the laboratory methods to be used in a soil background study is part of the USEPA DQO process, which is discussed in Section 8.2.
A soil background study, whether it is an existing study or one that will be conducted, should have a quality assurance plan. USEPA recommends having a quality assurance project plan (QAPP). This plan will specify the laboratory sample preparation method(s) and analytical method to be used for every analyte or analyte group. The quality assurance plan will also specify DQOs, such as measurement performance criteria (for example, various acceptable bias, precision, and analytical limit criteria) for every test method. Typically, the completeness (the number of analyses meeting all measurement performance criteria) for each analytical parameter and the entire analytical program are DQOs specified in the quality assurance plan. A complete list of laboratory DQO elements is provided by USEPA (USEPA 2002).
10.2.2 Test method bias and precision
For laboratory test methods, ASTM International provides definitions for bias and precision (ASTM E177 (ASTM 2019), ASTM E456-13A(2017)e4 (ASTM 2017)). Test method bias is “the difference between the expectation of the test results and an accepted reference value” ((ASTM 2017)), while test method precision is defined as a measure of “the closeness of agreement between independent test results obtained under stipulated conditions.”
The quality assurance plan will contain performance criteria that evaluate test method bias and precision for data generated by every test method. A number of QC samples will be evaluated for data quality indicators such as bias (for example, method blanks, matrix spikes, and laboratory control samples) and precision (for example, laboratory duplicates, matrix spike duplicates, and laboratory control sample duplicates). Surrogates will be used to indicate the potential bias in the analysis of individual samples for organic analytes. Results for these data quality indicators will be evaluated during the data validation stage (Section 10.2.4).
10.2.3 Laboratory quality system and analyte group accreditation
To ensure that high-quality analytical data with low test method bias and high test method precision are being generated, samples should be analyzed by laboratories whose quality systems have been accredited. Examples of items that are included in laboratory quality systems are their processes for calibration, calibration verification, and laboratory quality control.
Environmental laboratories are generally accredited to ensure that their quality system meets NELAC (National Environmental Laboratory Accreditation Conference) requirements (NELAC 2016). NELAC requirements meet the quality system requirements for ISO Standard 17025 (ISO 2017), in addition to country-specific requirements for the United States. Laboratories are accredited by accreditation bodies (either state regulatory agencies or select nongovernmental organizations) that perform on-site quality system assessments. Note that a state or stakeholder agency (for example, the Department of Defense) may have their own laboratory accreditation programs and/or requirements.
An accredited quality system means that the system has met certain minimum standards; it does not ensure that for individual test methods, the data reported by the laboratory have minimal test method bias. However, an accredited quality system makes this outcome more likely. It should be noted that as part of many accredited quality systems, the laboratory provides an estimate of the measurement uncertainty for every analytical test method in the laboratory.
Proficiency testing programs are offered to the laboratory by the accreditation agency and involve the regular analysis of samples with an unknown concentration. The laboratory must participate in the proficiency testing program for any analyte groups for which it wishes to be accredited. If results for a proficiency testing sample are outside the acceptance limits (that is, the test method bias is unacceptably high or low), the laboratory can lose its accreditation for that analyte group. For soil background studies, the laboratory should be currently accredited (if accreditation is offered) for any analyte groups that are anticipated to be sent to the laboratory for analysis. Analysis should not be performed at a laboratory if the accreditation has been suspended for a specific analyte group, to avoid the generation of potentially suspect data.
10.2.4 Data validation
Data reported by an analytical laboratory need to be independently reviewed to assess whether the data are fit for the intended purpose (such as the comparison of background and site soil analytical data). Often, this review is performed using the data validation process. This process is an assessment of data quality, tracing the history of the sample from collection through sample storage, sample preparation, instrumental analysis, and data reduction; this process ensures that the resulting data from the sample’s analysis are accurate, traceable, and appropriately qualified if any data quality issues were discovered in the validation process.
Validation is a structured, documented review of the data. A qualified analytical chemistry data validation expert who is independent of the laboratory generating the data should perform this review, typically using USEPA data validation methodologies ((USEPA 2016), (USEPA 2017), (USEPA 2017)). Note that a state or stakeholder agency (for example, the Department of Defense) may have their own data validation requirements.
10.3 Analytical Limits
Environmental datasets often contain “nondetect” and/or “estimated” results, based on the limited sensitivity of laboratory methods used to measure contaminant concentrations (note that estimated results can often be based on other factors besides the limited sensitivity of laboratory methods). This sensitivity can be described using two general types of thresholds (depicted in Figure 10‑1)—the detection limit (DL) and the quantitation limit (QL). The reporting limit (RL), which is a surrogate for the QL, is also depicted in this figure. A review discussing detection, quantitation, and reporting limits in straightforward language has been published online by the American Industrial Hygiene Association (Brisson and Popp 2017). USEPA has also published an overview (USEPA 2006). A much more technically detailed review of analytical limits was the subject of an Advisory Committee on Water Information webinar (van Buuren 2017).
Figure 10‑1. Relationship between various analytical limits.
Source: Doug Blue (ExxonMobil) and Shahrokh Rouhani (NewFields).
Briefly, these analytical limits are described in more detail:
- Results that fall below the DL (termed nondetects) are indistinguishable from blank results.
- Nondetects are a form of censored data, referred to as left-censored data, because they are always reported as being less than the DL.
- Data that fall below the relevant DL are flagged (or “qualified”) by the analytical laboratory with a “U” code, which allows data users to identify such measurements.
- Most laboratory Certificates of Analysis will report the method detection limit (MDL) for a sample. Alternatively, the limit of detection (LOD) may be reported.
- USEPA does specify a methodology to set the MDL (this is detailed below).
- MDLs are laboratory-specific and instrument-specific; they can vary between different analytical instruments (even for the same manufacturer and model number) performing the same method in the same laboratory. However, many commercial laboratories use data pooled from all similar instruments, so MDLs are consistent within that instrument group.
- The treatment of results less than the MDL in statistical analysis of the data is discussed in detail in Section 11.3.
- Results that fall between the DL and QL are detected but are quantified with a higher degree of uncertainty.
- Values between the DL and QL are considered to be estimated.
- Data that fall between the relevant DL and QL are flagged (or “qualified”) by the analytical laboratory with a “J” code, which allows data users to identify such measurements.
- Most laboratory Certificates of Analysis do not report the QL for a sample; instead, an RL is reported.
- USEPA does not specify a methodology to set the QL.
- QLs are laboratory-specific and instrument-specific; the QL can vary between different analytical instruments (even for the same manufacturer and model number) performing exactly the same the method in the same laboratory. However, many commercial laboratories use data pooled from all similar instruments, so QLs are consistent within that instrument group.
- RLs are sometimes used by laboratories as a surrogate for the QL (see discussion below).
The definition of the MDL has evolved over time (USEPA 2007) and the definition and calculation methodology have recently changed. The MDL is now defined as “the minimum measured concentration that can be reported with 99% confidence that the measured concentration is distinguishable from method blank results” (USEPA 2016). The MDL accounts for aspects of measurement such as the instrumentation, sample preparation, matrix effects, and laboratory reagents. The limit of detection (LOD) is the measure of an analytical method to detect the presence of an analyte with a 99% level of confidence; it does not provide information on the quantification of an analyte. The LOD is a concept similar to the MDL, in that it is a measure of the DL, although it is calculated differently than the MDL.
Instead of a QL, a laboratory will sometimes report an RL on their Certificates of Analysis. Like a QL, the RL is the lowest concentration of analyte in a sample that can be reported with a defined degree of analytical test method bias and precision. The USEPA does not specify a methodology to set the RL and there are multiple definitions of the RL (van Buuren 2017). Often, the laboratory sets the RL value at the QL, plus an added margin of safety to account for variations in the test method that may occur over time, or variation in instrument performance. However, in some cases, the laboratory will set the RL equal to the QL, which is why the RL is shown to have a range of possible values in Figure 10‑1. For example, consider a laboratory that has three instruments (same model and manufacturer) performing metals analysis for soil samples. For copper, the QLs for the three instruments are determined to be 3.7 mg/kg, 3.8 mg/kg, and 4.1 mg/kg. In this case, the laboratory may set the RL for copper to 5 mg/kg to avoid having to report different QLs for different instruments on the Certificate of Analysis.
For soil background studies, it is important that the analytical methods used meet the project DQOs. The RLs of the analytical methods used must meet the project DQOs, so they will be low enough to detect and quantify the analytes of interest, as well as minimizing (to the extent possible) left-censoring of the data. For many contaminants (especially organic contaminants), background concentrations may be very low. To minimize the number of data points less than the RL in the background dataset for these contaminants, it is often required to use “low-level” test methods (the test method that provides the lowest reporting limits for the analytes).
Typically, reported results greater than the DL are used “as is” (even if results are estimated) when calculating soil background values. There are several recommended procedures for treatment of data less than the DL when calculating soil background values (see Section 11.3 for further discussion).
Accredited laboratories should ensure sample preparation and analytical methods can generate the appropriate MDL and QL or RL, as per the project-specific DQOs. Early coordination with the selected laboratory can ensure that project objectives are met; the laboratory should review the project quality assurance plan to ensure accuracy and achievability before samples are submitted for analysis. Note that a state or stakeholder agency (for example, the Department of Defense) may have their own detection limit requirements.
10.4 Sample Preparation
Sample preparation broadly covers the procedures performed on the soil sample from the time of its receipt by the laboratory up until instrumental analysis is performed. Organic analytes are typically extracted from the soil using an organic solvent and it is the extract that is instrumentally analyzed. For metals, the soil sample is digested using acids and it is this digestate that is instrumentally analyzed. However, soil samples typically require preprocessing before extraction or digestion; this preprocessing is also considered part of sample preparation. This preprocessing can include steps such as sample drying (air, oven, or chemical), disaggregation, sieving and milling, or pulverizing, as well as subsampling of the preprocessed soil to obtain an aliquot for digestion or extraction.
Typically, the largest variability in the reported results is due to the sample preparation methods used for the soil sample, not the analytical method used to obtain the reported result. Different sample preparation methods can produce very different results for the same sample, so results may not be comparable if different sample preparation methods are used (this may also be true if different analytical methods are used). For that reason, common sample preparation methods for metals and various organic analyte groups are discussed in Table 10‑1; the table gives a brief synopsis of the sample preparation method (including details on sample preprocessing and extraction or digestion details) and also discusses whether the sample preparation method is suitable for use in soil background studies. For a more detailed description of the sample preparation methods, the reference methods identified in Table 10‑1 should be consulted.
Note that many USEPA SW-846 methods are referenced in Table 10‑1 and Table 10‑2. The USEPA periodically updates methods and changes the letter after the numerical method designation to reflect this revision. In general, use of the newest revision of each method is recommended, though there might be project-specific reasons to use an older version of a method. To facilitate the application of this guidance over time, the revision letters have been omitted from the method numbers in these tables. However, project-specific documents should always clearly identify the applicable revision number being used.
Soil sample preparation considerations when compiling soil background datasets include:
- For metals, soil sample preparation differs, depending on whether the goal is to determine the total metal concentrations in the sample, or just the environmentally available concentration of these metals. Sample digestion for total metals typically involves the use of hydrofluoric acid to more fully dissolve the aluminosilicate soil matrix and liberate more metals. It is harsher than the sample digestion procedures for environmentally available metals. Thus, greater soil metals concentrations will be obtained if the same sample undergoes total metals digestion versus digestion for environmentally available metals. For risk assessment purposes (see further discussion in Section 4), it is the environmentally available concentration of metals that should be quantified, not the total concentration.
- For example, the USGS performed a low sample density study (one sample per 1,600 km2) to determine elemental soil background values across the conterminous United States (Smith et al. 2014). USGS used a total metals digestion of the <0.15 mm fraction using four acids (including hydrofluoric acid) as the digestion method, which yields higher concentrations for metals than the less aggressive USEPA digestion methods used to determine environmentally available concentrations. Metals data from USGS studies (where total metals are quantitated) cannot be directly compared to data generated for environmentally available metals using USEPA methods, so they should not be used in soil background studies (Brooks 2020).
- For metals analysis, sample digestion targeting just environmentally available metals (USEPA Method 3050 or USEPA Method 3051) can give reported concentrations up to an order of magnitude less than when a more aggressive total metals digestion method (USEPA Method 3052) is used for the same sample (Ames and Prych 1995). Soil samples digested using the USGS methodology will give even higher metal concentrations results than USEPA Method 3052, since it uses a more aggressive acid digestion and analyzes either the <2 mm or <0.15 mm soil fraction (see discussion in next bullet).
- Critically, different sample preprocessing and/or digestion methods (but using the same analytical instrumentation) can give a much larger difference in the reported metals results for a soil sample versus if the same soil sample underwent the same sample preparation procedures but was analyzed using inductively coupled plasma (ICP)/mass spectrometry (MS) rather than ICP/atomic emission spectroscopy (AES) instrumentation.
- Sample preprocessing can affect the reported concentrations of environmentally available metals. USEPA digestion methods do not specify what preprocessing is to be performed, so different laboratories use different options. Some of these options (and their influence on the reported soil metals concentrations) are discussed in detail below (note that this list does not include all possible preprocessing options):
- Option 1—a wet soil sample typically just has the largest stones manually picked out of the sample and the sample is digested. The metals concentrations for these samples are calculated on a dry weight basis using results of the moisture analysis of that sample. With no sample preprocessing that affects the particle size distribution of the soil sample being digested for analysis, this option will provide the lowest environmentally available metals concentrations for the soil sample.
- Option 2—a wet soil typically has the largest stones manually picked out of the sample. The soil sample is air dried (or dried in an oven at 30°C) and disaggregated (broken apart), and dry sample is digested. With no sample preprocessing that affects the particle size distribution of the same sample being digested for analysis, this option will provide environmentally available metals concentrations for the soil sample similar to Option 1.
- Option 3—a wet soil has the largest stones manually picked out of the sample. The soil sample is air dried (or dried in an oven at 30°C) and disaggregated. The disaggregated sample is passed through a 10-mesh (<2 mm) sieve and the fraction passing through the sieve is digested. This sample preprocessing results in soil with smaller particle sizes and higher surface area being digested for analysis. This option will provide environmentally available metals concentrations for the soil sample directionally higher than Options 1 and 2.
- Option 4—a wet soil has the largest stones manually picked out of the sample. The soil sample is air dried (or dried in an oven at 30°C) and disaggregated. The sample is passed through a 10-mesh (<2 mm) sieve, with the fraction passing through this sieve then further milled/pulverized. After milling, the sample is passed through a 100-mesh (<0.15 mm) sieve and the fraction passing this sieve is digested. This sample preprocessing results in soil with even smaller particle sizes and higher surface area being digested for analysis than for Option 3. This option will provide environmentally available metals concentrations for the soil sample directionally higher than Option 3.
- Soil sample preprocessing methodologies used prior to digestion are a key factor to consider when determining whether the metals results from two datasets are substantively the same or not.
- Sample preprocessing methods should be tailored to fit the intended use of the analytical data. For example, pulverizing of soil is generally not appropriate when the dermal exposure pathway is being evaluated.
- For organic contaminants, sample preparation involves the extraction of the target analytes from the soil sample. The concentration of analyte obtained from analysis can vary widely, depending on the solvent chosen to extract the analytes from the soil matrix. Generally, organic analyte data from datasets generated using two different extraction solvents should not be combined in the same background dataset.
- In addition to extraction solvent, if different methods are used to clean up the solvent extract before analysis, this can influence the reported results. The magnitude of this influence is typically less than the size of the effect observed from using different extraction solvents.
- Incremental sampling methodology (ISM) can be applicable for background studies. The ITRC ISM-2 guidance ((ITRC 2012), (ITRC 2020)) describes the sample preprocessing options of air drying, disaggregation, sieving, milling, and two-dimensional slabcake subsampling. See Section 5 of the ISM-2 guidance for details on using project objectives to select among the sample preprocessing options if ISM is being used and how to implement these options at the laboratory.
- To completely understand the differences in sample preparation (especially sample preprocessing before digestion or extraction) between the laboratories used in two studies, just comparing the reference methods used will not suffice, since there is often some flexibility provided in the reference method. It is recommended that the standard operating procedures used by both laboratories be examined in detail to see if sample preparation methods could result in substantive differences in the reported results between the two studies. Standard operating procedures are typically appended to the QAPP (if the QAPP is available for review) or can be provided (upon request) by the laboratory that performed the sample analysis.
Table 10‑1. Sample preparation
Sources: (USEPA 2020) and (Taggart 2002).
| Chemical | Reference Method | Summary | Comments |
| Metals | USEPA Method 3050 (Heating Block Digestion) | Soil is preprocessed using a number of options (see the text of Section 10.4 for a full discussion). The preprocessed soil is digested at 90–95°C on a hot plate or heating block. Digestion uses nitric acid, hydrogen peroxide, and typically hydrochloric acid (HCl always used for ICP/AES and can be used with some ICP/MS systems). | Suitable for soil background studies. Will dissolve all environmentally available metals, but not aluminosilicate-bound metals that are not environmentally available. |
| USEPA Method 3051 (Microwave Digestion) | Mimics USEPA 3050B, except it uses microwave heating of sample and hydrogen peroxide is not used. | Suitable for soil background studies. Same comments as for 3050; provides similar results as 3050. Has shorter digestion times than 3050 and higher precision (better temperature control, versus heating block). | |
| USEPA Method 3052 (Total Digestion) | Similar to USEPA 3051, except the sample is microwave-digested at 180±5°C, using a mixture of nitric and hydrofluoric acids. Goal is the total decomposition of the sample, including all aluminosilicate and organic matrices. Analysis of the digestate yields a total metals value. | Not suitable for soil background studies. Dissolves all metals, including silicate-bound metals that are not environmentally available. Provides soil metals results biased high compared to USEPA Method 3050 and USEPA Method 3051. | |
| USGS Q030, T01 & T20 (Total Digestion) | Soil is air dried; fraction passing through a 10-mesh sieve (the <2 mm fraction) is retained for analysis. Optionally, the <2 mm fraction can be further pulverized and only the fraction passing through a 100-mesh sieve (<0.15 mm fraction) is retained for analysis. The sample is digested with four acids (hydrochloric, nitric, perchloric, and hydrofluoric) on a hot plate or heating block for several hours at temperatures up to 160°C. The goal is the total decomposition of the sample, including all aluminosilicate and organic matrices, to yield a total metals value. | Not suitable for soil background studies. Dissolves all metals, including silicate-bound metals that are not environmentally available. Provides soil metals results biased high compared to USEPA digestion methods for environmentally available metals (USEPA Method 3050 and USEPA Method 3051). | |
| Mercury | USEPA Method 7471 (Mercury Digestion) | Sample digested in 3:1 HCl:HNO3 (aqua regia); oxidized with potassium permanganate. | Suitable for soil background studies. Will dissolve all environmentally available mercury. |
| OCP, PAH, PCB, PCDD/F TPH | USEPA Method 3540 (Soxhlet Extraction) | A soil sample is chemically dried with anhydrous sodium sulfate, placed in a thimble, and extracted using the appropriate solvent in a Soxhlet extractor. If necessary, the extract is further processed (for example, dried, concentrated, and cleaned up) before analysis. | Suitable for soil background studies. Rigorous and rugged reference method to which all other methods are compared. Uses large solvent volume and long extraction time (16–24 hours). Preparation methodologies with shorter extraction times are more typically used and provide substantively equivalent results that are suitable for background studies. |
| USEPA Method 3541 (Automated Soxhlet Extraction) | Soil sample dried with anhydrous sodium sulfate is immersed in boiling solvent, then Soxhlet extracted (similar to USEPA 3540) and finally concentrated. If necessary, the extract is further processed (for example, dried, concentrated, and/or cleaned up) before analysis. | Suitable for soil background studies. Uses shorter extraction times (~2 hours) and smaller solvent volume than USEPA Method 3540, while still giving analyte recoveries similar to that method. | |
| USEPA Method 3545 (Pressurized Fluid Extraction) | Chemically dried soil sample is placed in extraction cell and heated (temperature and time depend on analyte). Sample is pressurized (1,500–2,000 psi) with appropriate solvent. Multiple extraction cycles used for some analytes. If necessary, the extract is further processed (for example, dried, concentrated, and/or cleaned up) before analysis. | Suitable for soil background studies. Typical extraction cycle is 5–10 minutes. Small solvent volumes used in extraction. | |
| USEPA Method 3546 (Microwave Extraction) | Chemically dried soil sample is placed in extraction cell with the appropriate solvent and heated via microwave (temperature and pressure depend on the analyte). One extraction cycle used. The extract is filtered to remove solids. If necessary, the extract is further processed (for example, dried, concentrated, and/or cleaned up) before analysis. | Suitable for soil background studies. Uses shorter extraction times (10–20 minutes) and smaller solvent volume than USEPA Method 3540, while still giving analyte recoveries similar to that method. | |
| OCP, PAH, PCB, PCDD/F TPH | USEPA Method 3550 (Ultrasonic Extraction) | Chemically dried soil sample is placed in an ultrasonic cell with the appropriate solvent and the mixture is extracted with solvent three times. The extract is separated from the soils by filtration or centrifugation. If necessary, the extract is further processed (for example, dried, concentrated, and/or cleaned up) before analysis. | Not suitable for soil background studies. Uses shorter extraction times than USEPA 3540, but still uses relatively large solvent volumes. Method states it “might not be as rigorous as other extraction methods for soils” and that recoveries for some analytes are low. Not recommended for environmental soil background studies, due to potential for low recoveries for some analytes. |
| VOCs | USEPA Method 5035 (Purge and Trap) | The methanol preservation option, with a 1 g soil to 1 mL methanol ratio in a preweighed vial, is recommended for best analyte recovery. An aliquot of the extract is diluted in reagent water and purged onto the trap of the analytical instrument. The low concentration options using aqueous preservation solutions can produce low biased results when the VOCs are strongly bound to the soil particles. | Suitable for soil background studies. Reporting limits are typically higher for methanol-preserved samples than the low-level options. The methanol option is preferable for soil background studies, if the analysis is performed with enhanced mass spectrometer sensitivity (for example, using selected ion monitoring) to compensate for the required dilution of the methanol extract. |
Notes:
OCP—organochlorine pesticides
PAH—polycyclic aromatic hydrocarbons
PCB—polychlorinated biphenyls
PCDD/DF—polychlorinated dibenzo-p-dioxins/dibenzofurans
TPH—total petroleum hydrocarbons
VOC—volatile organic compounds
10.5 Analytical Test Methods
For common metals and various organic analyte groups for contaminants of concern at sites, the analytical test methods used are discussed in Table 10‑2; the table gives a brief synopsis of the test method and discusses whether the analytical method is suitable for use in soil background studies. For a more detailed description of the analytical method, the reference method identified in Table 10‑2 should be consulted.
In risk assessments (including contaminant fate and transport modeling), analysis of soil samples for various other physical and chemical properties is useful, so collecting this data in a background study may be warranted. These parameters include (but are not limited to) grain-size distribution, pH, total organic carbon, and cation exchange capacity (Section 9.1.3). Discussion of the sample preparation and analytical test methods for these parameters is beyond the scope of this document.
Table 10‑2. Analytical methods
Sources: (USEPA 2020), (Taggart 2002).
| Chemical | Analytical Method(s) | Summary | Comments |
| Metals | USEPA Method 6010 USGS T01 (ICP/AES) | A digested sample is nebulized into an ICP, where the metal atoms are ionized. The metal ions are quantitated using AES. | Suitable for soil background studies if RLs are low enough. ICP/AES analysis is marginally less expensive than ICP/MS but has elevated RLs for some metals (for example, silver, thallium, and mercury). |
| USEPA Method 6020 USGS T20 (ICP/MS) | A digested sample is nebulized into an inductively coupled plasma (ICP), where the metal atoms are ionized. The metal ions are quantitated using mass spectrometry (MS). | Suitable for soil background studies. ICP/MS typically has lower RLs than ICP/AES, so use of ICP/MS is preferred for soil background studies (to lower the nondetect frequency for some trace metals). | |
| Mercury | USEPA Method 7471 (CVAA) | A digested sample is chemically reduced, converting divalent mercury to elemental mercury, which is aerated to vaporize the mercury. The cold vapor (CV) passes through an atomic absorption (AA) spectrometer, where the mercury is quantitated. | Suitable for soil background studies. Instrumentation is typically more readily available than ICP/MS. |
| OCP | USEPA Method 8081 (GC/ECD) | Sample is extracted using any of USEPA Methods 3540, 3541, 3545, or 3546. Extraction solvents typically used are 1:1 hexane/acetone or 1:1 methylene chloride/acetone. Extracts are cleaned up [for example, alumina (USEPA Method 3610), Florisil (USEPA Method 3620), or silica gel (USEPA (3630)]. After cleanup, the extract is analyzed by injecting into a capillary gas chromatograph, equipped with an electron capture detector (GC/ECD). | Suitable for soil background studies. Currently, GC/ECD use is preferred over GC/MS for soil background studies because of lower RLs. With GC/ECD, careful evaluation of low-level detections is recommended because of the potential for false positives. |
| PAH | USEPA Method 8270 (GC/MS) (Full Scan or Selected Ion Monitoring (SIM) mode) | Sample is extracted using any of USEPA Method 3540, 3541, or 3545. Methylene chloride/acetone is the extraction solvent typically used, with extract cleanup typically not performed. The extract is analyzed by injection into a capillary gas chromatograph, equipped with mass spectrometer detector (GC/MS) operated in either full scan or SIM mode. | Suitable for soil background studies. Price difference between full scan and SIM analysis is small. In soil background studies it is preferred to use methods with lower reporting limits (for example, SIM). |
| PCB | USEPA Method 8082 (GC/ECD) | Sample is extracted using any of USEPA Methods 3540, 3541, 3545, or 3546, using 1:1 hexane/acetone or 1:1 methylene chloride/acetone. Extracts are cleaned up using sulfuric acid/potassium permanganate (USEPA Method 3665). After cleanup, the extract is analyzed by injecting into a capillary gas chromatograph, equipped with an electron capture detector (GC/ECD). | May be suitable for soil background studies. Much less expensive than USEPA Method 1668. Only quantitates Aroclors and select congeners. Higher RLs than congener analysis. Suitable for soil background studies, if data for all congeners not needed and RLs meet DQOs. |
| USEPA Method 1668 (HRGC/HRMS) | Sample is typically extracted using USEPA Method 3540, using hexane as the extraction solvent. Extracts are cleaned up [for example, Florisil (USEPA Method 3620)]. After cleanup, the extract is analyzed by injection into a high-resolution gas chromatograph, equipped with a high-resolution mass spectrometer detector (HRGC/HRMS). | Suitable for soil background studies. Much more expensive than USEPA Method 8082. Can individually detect most congeners at lower levels than Aroclor analysis. | |
| PCDD/DF | USEPA Method 8290 (HRGC/HRMS) | Sample is extracted using USEPA Method 3540 or USEPA Method 3545 using toluene as the solvent. Extracts then acid/base washed, dried, and cleaned up using a column containing alumina, silica gel, and activated carbon. After cleanup, the extract is analyzed using a high-resolution gas chromatograph, equipped with a high-resolution mass spectrometer detector (HRGC/HRMS). | Suitable for soil background studies. Able to detect PCDD/DF congeners at very low levels (ng/kg levels). |
| TPH (GRO and DRO) | USEPA Method 8015 (GC/FID) | For DRO, sample is extracted using any of USEPA Methods 3540, 3541, 3545, or 3546, typically using a methylene chloride/acetone solvent that is cleaned up with silica gel (USEPA Method 3630) when removal of polar sample components is appropriate. For GRO, purge & trap (USEPA Method 5035) or static headspace (USEPA Method 5021) are often used. Quantitation is via capillary gas chromatograph, equipped with a flame ionization detector (GC/FID). Gasoline range organics (GRO) quantitates C6-C10, while diesel range organics (DRO) typically quantitates C10-C28. | Suitable for soil background studies. RLs less than USEPA Method 1664 (gravimetric determination of hexane extractable materials) are possible. |
| VOC | USEPA Method 8260 (GC/MS) (Full Scan or SIM mode) | Purge & trap (USEPA Method 5035) or static headspace (USEPA Method 5021) are often used. The vapor generated from the sample is analyzed using a capillary gas chromatograph, equipped with mass spectrometer (GC/MS) operated in either Full Scan or SIM mode. | Suitable for soil background studies. Price difference between full scan and SIM analysis is negligible. Methods with lower RLs, such as SIM, are preferable. |
Notes:
DRO—diesel range organics
GRO—gasoline range organics
OCP—organochlorine pesticides
PAH—polycyclic aromatic hydrocarbons
PCB—polychlorinated biphenyls
PCDD/DF—polychlorinated dibenzodioxins/dibenzofurans
TPH—total petroleum hydrocarbons
VOC—volatile organic compounds
Analytical method considerations when compiling soil background datasets include:
- Soil results for organic and inorganic methods are typically reported on a dry weight basis. However, in some studies (especially older studies) results might be reported on a wet weight basis. If results were reported on a wet weight basis, convert these values to a dry weight basis before including them in a background dataset. If it is not possible to perform this conversion (there is no soil moisture analysis available for the sample for which data were reported on a wet weight basis), the wet weight data should not be used in a background dataset containing dry weight values.
- Metals field screening data generated using portable x-ray fluorescence (XRF; USEPA Method 6200) should not be included in the background dataset generated by the laboratory using ICP/AES, ICP/MS, or cold vapor atomic absorption (CVAA) spectroscopy methods. These field XRF results do not have the strict QA/QC used to generate the environmentally available metals data in the laboratory; quantitation is based on a method with entirely different sample preparation. In addition, XRF quantitates total metals and not the environmentally available metals concentrations considered in soil background studies. However, portable XRF data can be useful to field-screen soil samples, to select samples to be submitted for laboratory analysis for metals.
- Note that laboratory-based XRF instruments do generate data under strict QA/QC protocols. However, because XRF is a measure of the total metals concentration in the soil sample, laboratory data generated from this analytical method should not be included in background datasets for environmentally available metals.
- If a geochemical evaluation to establish soil background is planned, refer to Section 5 for guidance on the necessary metals to include in the analysis. USEPA’s target analyte list (TAL) of 23 metals contains all the reference elements (aluminum, iron, manganese, calcium, magnesium, sodium, and potassium) and most of the trace elements (antimony, arsenic, barium, beryllium, cadmium, chromium, cobalt, copper, lead, mercury, nickel, selenium, silver, thallium, vanadium, and zinc) needed to perform a geochemical evaluation. In some cases, additional trace metals (for example, molybdenum) may need to be added to this list, if these elements are COPC in the soil background study.
- Some jurisdictions have total PCB criteria for soils. Total PCB values calculated from the Aroclor data (USEPA Method 8082) will give a different result (typically lower) than the total PCB concentrations calculated using congener data (USEPA Method 1668). When determining background values for total PCB, the mixing of data generated using these two test methods in the background dataset should be avoided.